- Enhanced Bio-Electrodegradation of Acetylsalicylic Acid: Optimization, Pathway Elucidation, and Environmental Application
Harshavardhan Mohan, Pavithra Muthukumar Sathya, Min-Ju Kim, Bongkyu Kim*, and Byung-Taek Oh*
Division of Biotechnology, Advanced Institute of Environment and Bioscience, College of Environmental and Bioresource Sciences, Jeonbuk National University, Iksan, Jeonbuk State 54596, Republic of Korea
- 아세틸살리실산의 향상된 생물전기분해: 최적화, 분해 경로 규명 및 환경적 적용
하샤 발단모한ㆍ무투쿠마르 사티아 파비트라ㆍ김민주ㆍ 김봉규*ㆍ오병택*
전북대학교 생명공학부
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
The widespread use and environmental persistence of acetylsalicylic acid (ASA), a commonly consumed pharmaceutical, necessitate the development of efficient and sustainable removal strategies. This study investigates the degradation of ASA using a bio-electrochemical system (BES) employing Pseudomonas putida ASP-7, isolated from animal hospital waste. Comparative assessments revealed that bio-electrodegradation achieved significantly higher removal efficiency (98.7%) than biodegradation (59.54%) and electrodegradation (12.55%), with superior degradation kinetics and half-life. Optimization experiments identified ideal conditions at 20 mV applied potential, pH 6.5, and 26-30 °C using 100 mg/L ASA. Metabolic analysis showed ASA was initially hydrolyzed to salicylic acid and catechol, which further degraded into non-toxic end products. When applied to ASA-spiked river water, the system achieved 87.51% removal, demonstrating its practical potential for real-world pharmaceutical pollutant remediation.
Keywords: Acetylsalicylic acid, Bio-electrochemical system, Degradation, Metabolic pathway, Pseudomonas putida
Acetylsalicylic acid (ASA), widely recognized as aspirin, ranks among the most extensively used pharmaceutical agents worldwide, primarily due to its therapeutic roles in alleviating pain, reducing fever and inflammation, and preventing blood clots (Mohan et al., 2021; Shangguan et al., 2025). Environmental contamination by ASA arises through several routes, including partial excretion by humans and animals, improper disposal practices, and the release of effluents from healthcare facilities, pharmaceutical manufacturers, and sewage treatment plants (F. Wang et al., 2024). The continuous release and persistence of ASA in the environment raise growing concerns due to its potential ecological and toxicological impacts. Even at low concentrations, ASA and its by-products can disturb microbial ecosystems, disrupt endocrine systems in aquatic organisms, induce genetic damage, and alter the behavior of aquatic fauna (Park et al., 2023; Huan Wang et al., 2021). In addition to aquatic impacts, ASA and other pharmaceutical residues can reach soil and groundwater through pathways such as wastewater discharge and infiltration, leakage from sewer infrastructure, irrigation with reclaimed water, and percolation from contaminated surface waters (Hui Wang and Zhou, 2024). In these contexts, effective treatment at point sources (e.g., hospital and pharmaceutical effluents or concentrated side-streams) is critical to reduce mass loading into subsurface environments and mitigate long-term exposure risks (F. Wang et al., 2024).
Several remediation technologies have been explored to mitigate pharmaceutical and ASA pollution, including biological treatment processes (Shangguan et al., 2025), advanced oxidation processes (AOPs) (Matamoros, García, & Bayona, 2008), membrane separation, and adsorption techniques (Sophia, 2018). Despite their utility, these conventional approaches are often hindered by limitations such as high operational costs, low efficiency against stable pharmaceutical residues, the formation of toxic intermediates, and secondary waste generation (Chinnasamy et al., 2023; Saravanan et al., 2022). To address these limitations, bio-electrochemical systems (BES) have emerged as a novel and eco-efficient approach for pharmaceutical and polycyclic aromatic hydrocarbon degradation (Sathya, Mohan, Park, Seralathan, Cho, et al., 2024). BES harness the combined power of electrochemical input and microbial metabolism to accelerate pollutant breakdown, offering higher removal efficiencies, reduced energy consumption, and a smaller environmental footprint (Sathya, Mohan, Park, Seralathan, & Oh, 2024; S. Wang, Hadji-Thomas, Adekunle, & Raghavan, 2024). In particular, bio-electrodegradation where microbial activity is enhanced by an applied electric potential shows great promise for the sustainable and effective elimination of persistent contaminants like ASA, surpassing the performance of many conventional treatment methods (Moghiseh & Rezaee, 2021; Mohan et al., 2024).
Therefore, this study aims to: (1) isolate and identify effective ASA-degrading bacteria from animal hospital waste; (2) conduct a comparative evaluation of different ASA degradation approaches; (3) optimize key experimental parameters to achieve maximum degradation efficiency; and (4) elucidate the degradation pathway of ASA.
2.1. Chemicals
The chemicals utilized in this study were procured from Sigma-Aldrich, South Korea, and used as received without further purification.
2.2. Isolation, Identification and MIC of potential bacterial strain
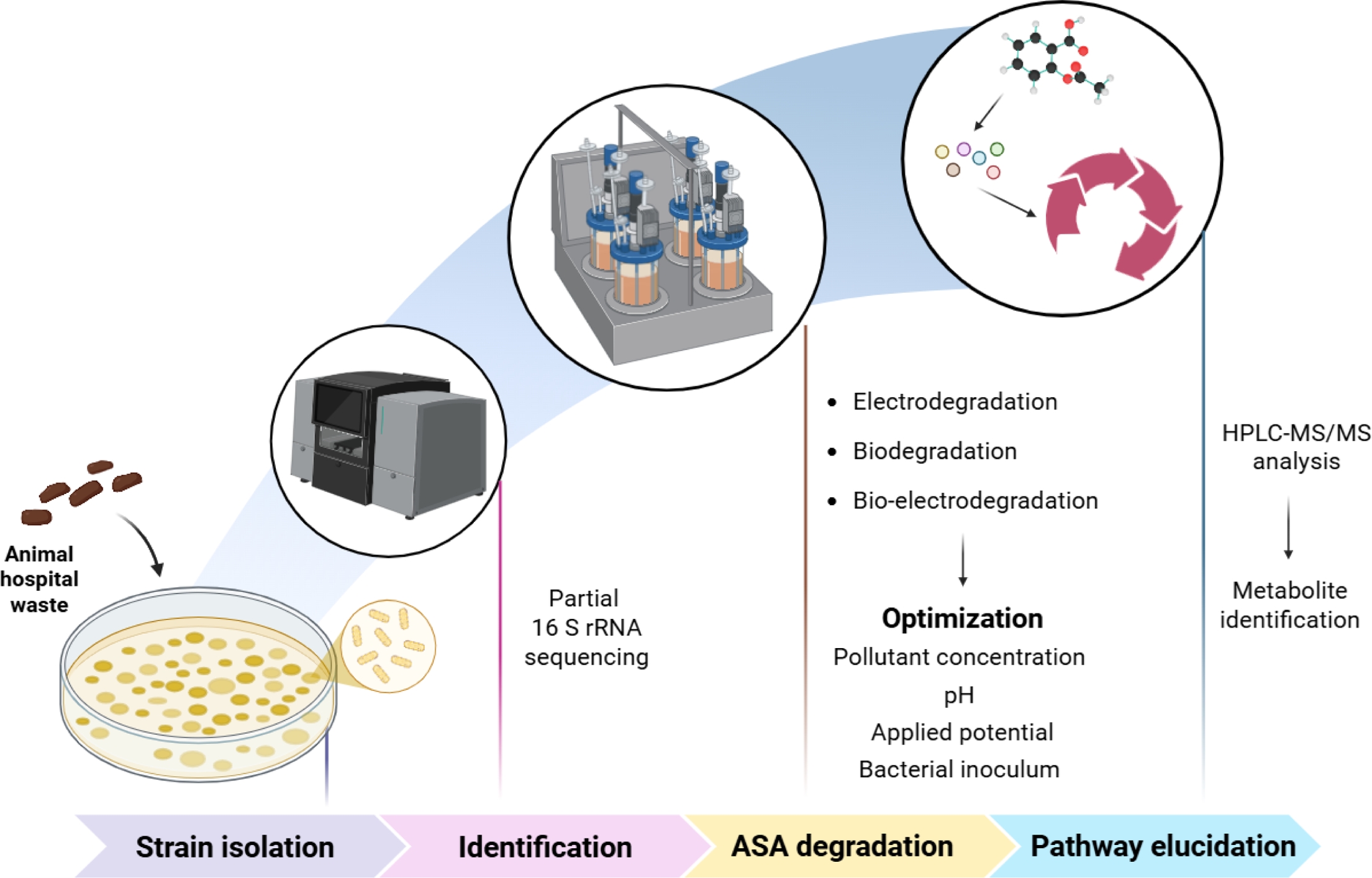
Bacterial strains capable of degrading ASA were isolated from animal hospital waste in Iksan-si, South Korea. A 50 g sample was enriched in minimal salt medium (MSM) containing 20 mg/L of ASA and incubated at 30 ± 2°C for 24 h. After serial dilution (10-2 to 10-6) and plating on Luria-Bertani (LB) agar, distinct colonies were purified and screened for ASA tolerance based on optical density at 600 nm (OD600) measurements. The strain showing the highest growth was selected and preserved at -80°C. Strain identification was performed by 16S rRNA gene sequencing (primers 785f and 907r) followed by NCBI BLAST comparison and phylogenetic analysis using MEGA11. The minimum inhibitory concentration (MIC) of ASA was evaluated via a broth dilution assay in Mueller-Hinton Broth (pH 7.2 ± 0.2), with 20-300 mg/L ASA, and growth was measured at OD600 after 24 h at 30 ± 2°C. The schematic representation of the study is given in Fig. 1.
2.3. Degradation of ASA
ASA degradation was evaluated under three conditions: electrochemical (applied potential only), biodegradation (bacterial strain only), and bio-electrodegradation (microbes + electrodes + applied potential). A dual-chamber reactor (200 mL each) with stainless steel sheets (7 × 5 cm) electrodes and a proton exchange membrane was used. Initial conditions were 20 mg/L ASA, pH 7, temperature 30°C, 1% v/v inoculum, 10 mV applied potential, and a 10-day operation period. Samples were collected periodically and analyzed by HPLC-MS/MS (API3200, Applied Biosystems, USA). The percentage degradation of ASA was calculated using the formula:
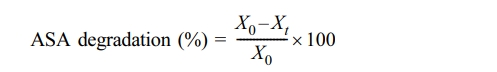
where X0 represents the initial concentration of ASA and Xt is the concentration at various time intervals. Optimization was performed by varying ASA concentration (50-250 mg/L), pH (6-8), applied potential (10-50 mV), and temperature (26-34°C). The optimization ranges were selected to reflect practical operating windows for bio-electrochemical treatment of near-neutral water matrices while maintaining stable microbial activity and enabling meaningful kinetic comparison. The pH range (6-8) was chosen to represent typical natural/treated waters and to avoid severe inhibition associated with acidic/alkaline stress. The temperature range (26-34°C) was selected to remain within a mesophilic window while assessing thermal sensitivity without destabilizing the biocatalyst. The ASA concentration range (50-250 mg/L) was selected to ensure reliable quantification and kinetic fitting across conditions and to represent a high-strength pharmaceutical loading scenario. The applied potential range (10-50 mV) was selected as a low-energy input window intended to promote bio-electrochemical electron transfer while minimizing parasitic reactions and unnecessary energy consumption. The degradation pathway was determined by identifying intermediates formed under optimal bio-electrodegradation process. Additionally, the applicability of the process was assessed using natural water collected from the Samcheon stream in Jeonju-si, South Korea, at the coordinates 35.843688°N latitude and 127.105063°E longitude, which was used by spiking it artificially with ASA for the study (Table 1).
|
Fig. 1 Schematic representation of the degradation of Acetylsalicylic Acid (ASA). |
|
Table 1 Physio-chemical properties of the natural water collected from Samcheon stream |
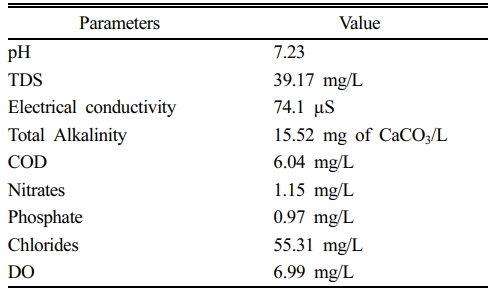
[All parameters were measured according to APHA Standard Methods.] |
3.1. Preliminary studies
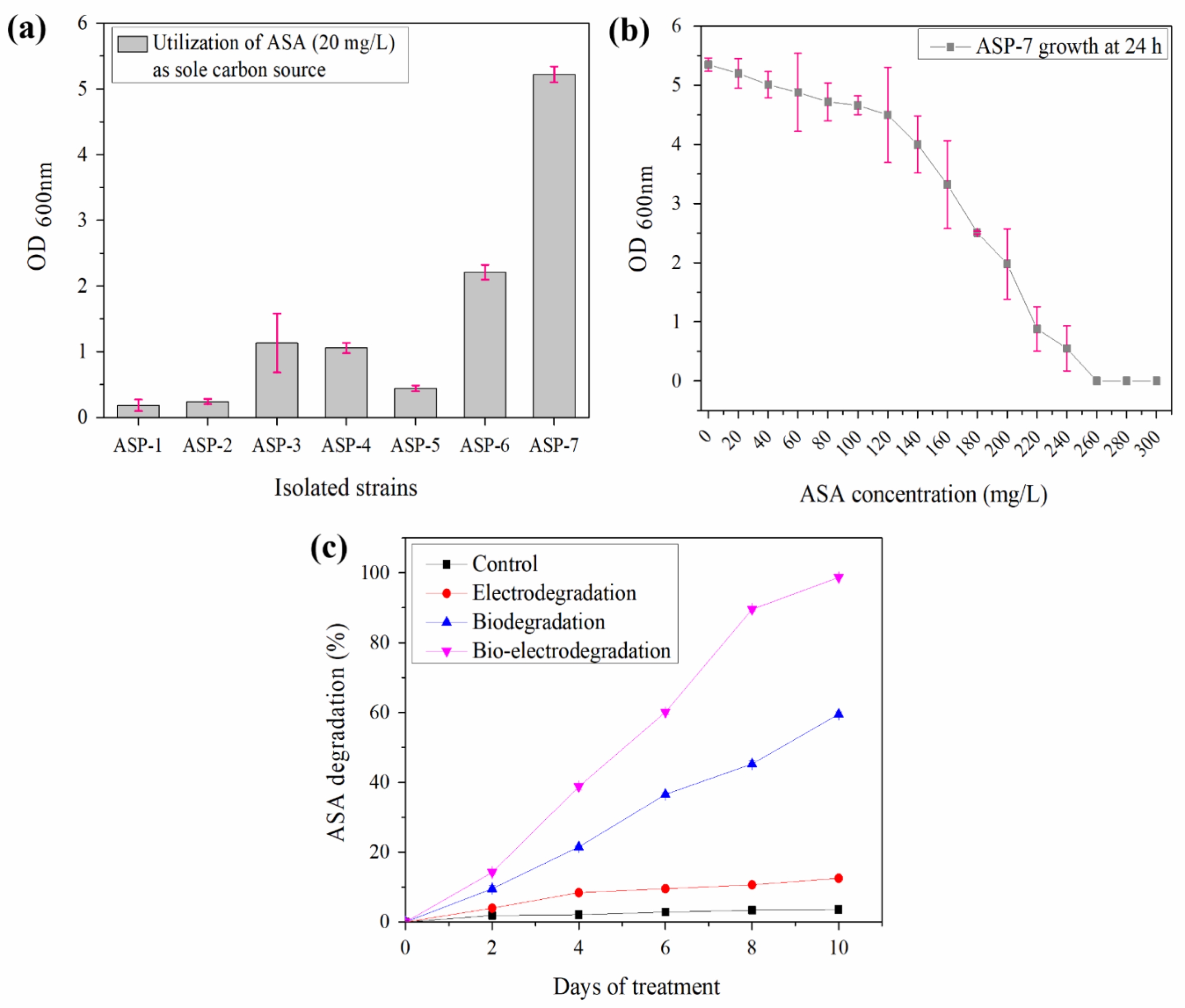
Seven ASA-utilizing bacterial strains (ASP-1 to ASP-7) were isolated, and strain ASP-7 showed the highest degradation capability (Fig. 2a). 16S rRNA sequencing identified ASP-7 as Pseudomonas putida with 99.75% similarity. Minimum inhibitory concentration (MIC) analysis revealed that P. putida ASP-7 exhibited optimal growth 20-140 mg/L ASA, while growth decreased at higher concentrations and was absent above 240 mg/L (Fig. 2b).
3.2. Degradation of ASA
Comparative experiments under electrodegradation (applied potential only), biodegradation (bacterial strain only), and bio-electrodegradation (combined bacterial strain and applied potential) showed distinct differences in ASA removal (Fig. 2c). Electrodegradation achieved 12.55% removal, biodegradation reached 59.54%, and bio-electrodegradation exhibited the highest efficiency at 98.7% with 10 days. All processes followed pseudo-first-order kinetics. The bio-electrodegradation exhibited the highest rate constant (0.1755 day-1), compared with biodegradation (0.0769 day-1) and electrodegradation (0.015 day-1), corresponding to half-lives of 3.95, 9.01, and 46.12 days, respectively (Table 2).
3.3. Experimental optimization
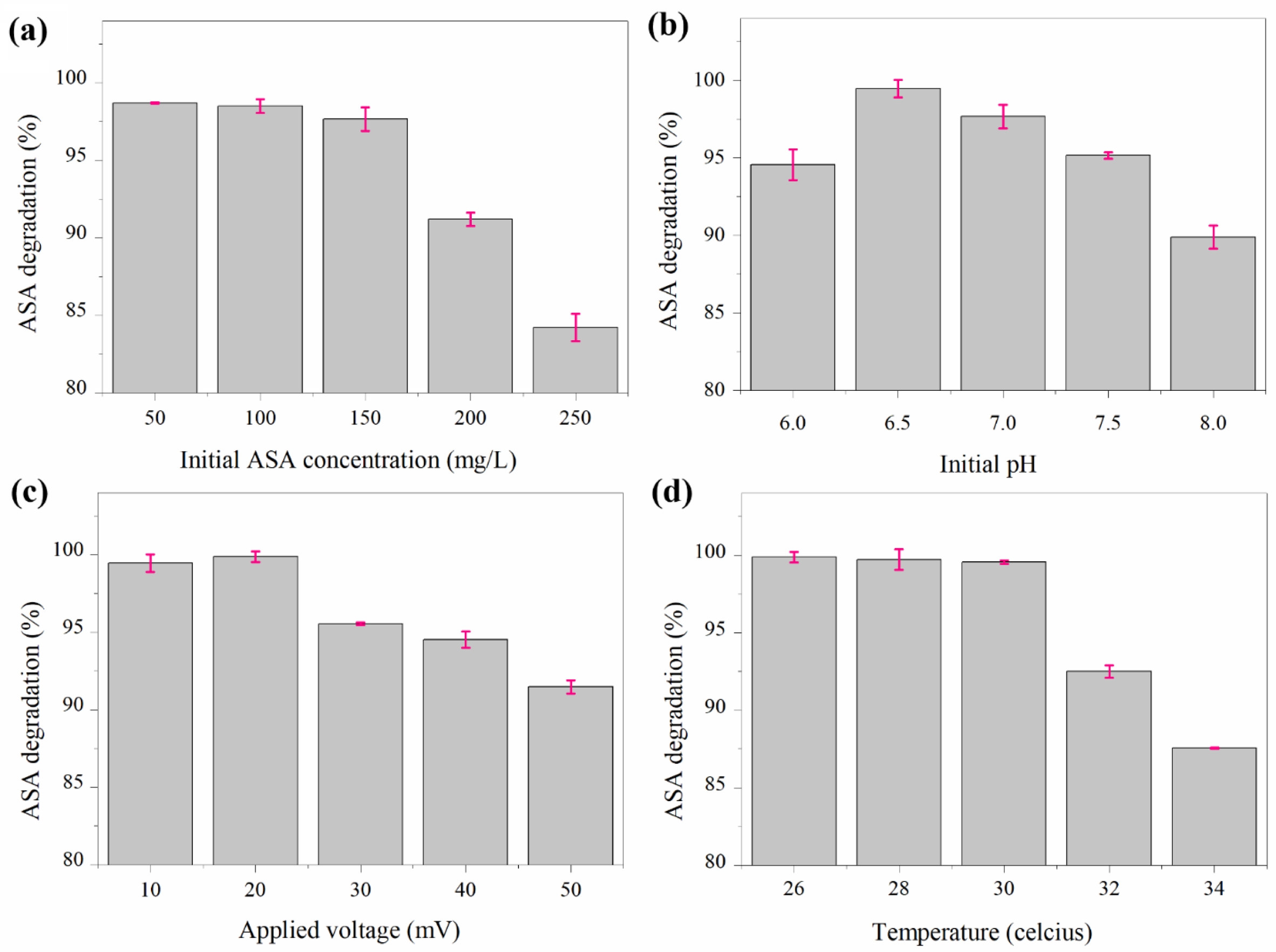
Optimization studies revealed that ASA bio-electrode- gradation remained highly efficient under favorable conditions. Degradation exceeded 98% at 50-100 mg/L ASA but gradually decreased to 84.2% at 250 mg/L (Fig. 3a). The influence of pH on degradation performance is illustrated in Fig. 3b. At pH 6.5 yielded the highest performance (99.45%), with reduced efficiency at both lower and higher pH values. An applied potential of 20 mV produced the maximum degradation (99.87%), whereas higher voltages resulted in declining performance (Fig. 3c). Temperature optimization showed that 26-30°C supported > 99.5% degradation, while efficiency decreased noticeably above 30°C (Fig. 3d).
3.4. Pathway of ASA degradation
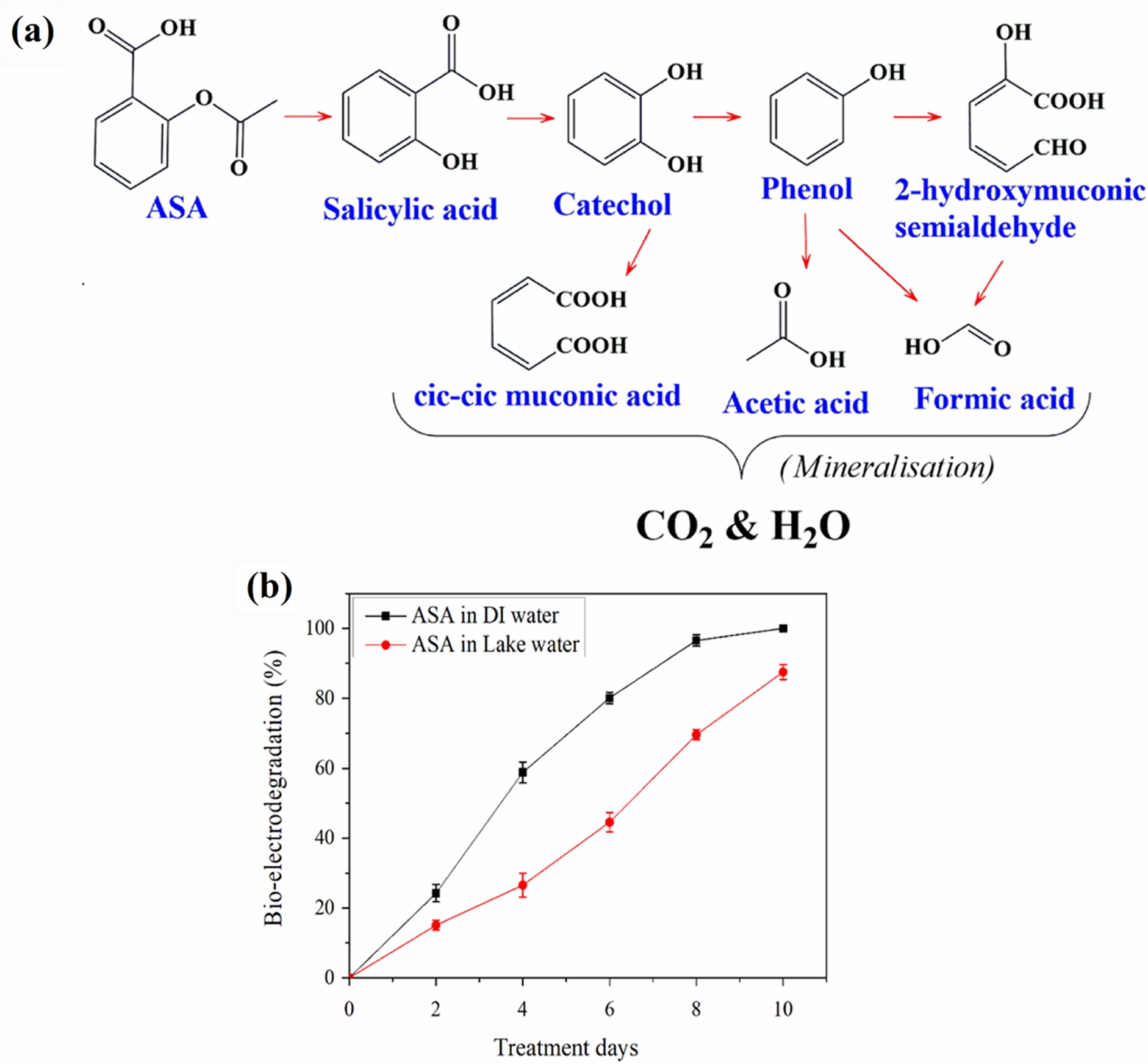
The metabolic pathway of ASA degradation by P. putida ASP-7 under optimized bio-electrodegradation conditions is depicted in Fig. 4a. ASA was first hydrolyzed to salicylic acid, which was further converted to catechol. Catechol proceeded through two pathways. First route, ortho-cleavage to cis,cis-muconic acid, leading to complete mineralized into CO2 and H2O. And second route, conversion to phenol, followed by transformation into 2-hydroxymuconic semialdehyde and subsequently into small aliphatic compounds such as acetic acid and formic acid, which were ultimately mineralized.
3.5. Environmental significance
To evaluate the practicality of the BES in natural water matrix, degradation tests were performed using river water. In DI water spiked with 100 mg/L of ASA, complete degradation (100%) was achieved, whereas the river water sample showed a slightly lower efficiency of 87.51% (Fig. 4b).
|
Fig. 2 (a) Isolation of potential bacteria capable of utilizing ASA as the sole carbon source, (b) Minimum Inhibitory Concentration (MIC) of ASP-7 against ASA and (c) Comparative study on the efficiency of electrodegradation, biodegradation and bio-electrodegradation of ASA. |
|
Fig. 3 Optimization of ASA bio-electrodegradation with respect to (a) Initial ASA concentration, (b) pH, (c) Applied potential, and (d) Temperature after 10 days of treatment. |
|
Fig. 4 (a) Elucidated ASA bio-electrodegradation pathway (Experimental conditions - ASA initial concentration: 100 mg/L; pH: 6.5; applied potential: 20 mV; temperature: 30°C; inoculum dose: 1% v/v) and (b) Bio-electrodegradation efficiency of ASA in artificially polluted river water. |
|
Table 2 Kinetics parameter for ASA degradation under different treatment methods |
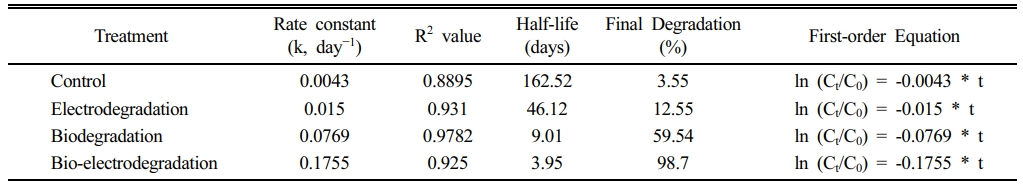
This study demonstrates the potential of a bio-electro- chemical system (BES) incorporating Pseudomonas putida ASP-7 for efficient ASA degradation. Among the seven strains isolated from pharmaceutical-contaminated hospital waste, ASP-7 exhibited the highest degradation capacity and tolerated ASA concentrations up to 240 mg/L. Its close genetic similarity to P. putida, a species well known for xenobiotic degradation, further supports its applicability for pollutant remediation (Mohan et al., 2020; Muthukumar Sathya, Mohan, Park, Seralathan, & Oh, 2023). When ASA degradation was evaluated under electrodegradation, biodegradation, and bio-electrodegradation condition, the bio-electrodegradation achieved the highest performance, with 98.7% removal and a significantly shorter half-life of 3.95 days. Pseudo-first-order kinetics were used to describe ASA removal because the experiments were performed in batch mode under constant operating conditions (electrode geometry/area, inoculum dose, mixing, pH, temperature, and applied potential), such that the apparent rate can be expressed as a function of ASA concentration alone. Under these conditions, the apparent rate constant (k) provides a practical basis for comparing treatment performance. The ln (Ct/C0) vs time (in days) data showed good linearity across treatments, supporting the use of the pseudo-first-order model within the tested conditions. The enhanced performance in the bio-electrodegradation can be attributed to the synergistic interaction between microbial metabolism and electrochemical stimulation, which likely accelerated electron transfer and intermediate breakdown at the electrode interface (Idris, Kim, Yaqoob, & Ibrahim, 2022; Kong, Li, Zhang, & Liu, 2023).
Optimization studies revealed key operational parameters influencing bio-electrodegradation performance. The system maintained high degradation efficiency (98.5%) at lower ASA concentrations (50-100 mg/L), but a decline was observed at higher concentrations, likely due to substrate inhibition or toxicity (Poddar et al., 2022; Sathya, Mohan, Venkatachalam, & Seralathan, 2023). The concentration window was intentionally selected to evaluate the robustness and tolerance of the bio-electrodegradation system under high-strength loading conditions and to enable reliable kinetic fitting and intermediate tracking without pre-concentration steps. Accordingly, the proposed approach is positioned for treatment of point-source or concentrated streams (e.g., hospital/industrial wastewater or side-streams) where higher pharmaceutical loads can occur in real effluents. The optimal pH was slightly acidic (pH 6.5), consistent with the physiological preference of many pseudomonas species and favourable for electrochemical activity (Mahgoub et al., 2023; Narayanan et al., 2022). An applied potential of 20 mV resulted in maximal degradation, while higher potentials may have induced electrochemical stress that compromised bacterial viability of biofilm stability (Almatouq, Babatunde, Khajah, Webster, & Alfodari, 2020; Tavker & Kumar, 2023). Temperature optimization indicated that 26-30°C supported the highest performance, whereas deviation from this range likely weakened enzymatic activity and reduced efficiency (Costa et al., 2019; Mahgoub et al., 2023).
The metabolic fate of ASA during bio-electrodegradation revealed a dual-pathway transformation mechanism. ASA was initially hydrolyzed to salicylic acid, followed by hydroxylation to catechol (Moghiseh, Rezaee, Ghanati, & Esrafili, 2019; Rocheleau, Al-Harthi, & Ouellet, 2019). Catechol underwent two degradation routes: ortho-cleavage to cis,cis-muconic acid and transformation to phenol, which was further broken down into 2-hydroxymuconic semialdehyde, and eventually into acetic and formic acids indicating progressive mineralization into non-toxic end products (Moghiseh et al., 2019; Mohan et al., 2021). Environmental relevance was validated by testing the system in stream water spiked with ASA, where a slightly reduced efficiency (87.51%) compared to DI water (100%) was observed. This slight decrease is likely due to the presence of natural organic matter and competing microbial populations. Nonetheless, the bio-electrodegradation maintained high degradation performance in a complex matrix, confirming its practical applicability for real-world wastewater treatment scenarios. Although this study was demonstrated in an aqueous BES reactor, the findings are relevant to soil/groundwater protection because controlling pharmaceutical loads at the source reduces downstream infiltration and soil exposure. In future work, we will validate performance in soil/groundwater-relevant setups (e.g., soil column leaching tests or groundwater microcosms) and assess matrix effects under subsurface-representative geochemistry.
This study investigates the bio-electrodegradation of acetylsalicylic acid (ASA) using a novel Pseudomonas putida strain (ASP-7) isolated from animal hospital waste. Among seven isolates, ASP-7 demonstrated the highest ASA degradation capacity and tolerance up to 240 mg/L. Bio-electrodegradation significantly outperformed biodegradation and electrodegradation, achieving 98.7% removal with enhanced kinetics (k = 0.1755 day-1). Optimal conditions included 100 mg/L ASA, 20 mV potential, pH 6.5, and 26-30°C. Pathway analysis revealed ASA transformation via salicylic acid and catechol, leading to complete mineralization through ortho-cleavage and formation of non-toxic intermediates. Application in river water spiked with ASA yielded 87.51% degradation, underscoring the practical relevance of the system. Validation at environmentally representative concentrations (μg/L-ng/L) in diverse real wastewaters will be addressed in future work. Overall, the results demonstrate the effectiveness and environmental applicability of bio-electrochemical systems for pharmaceutical pollutant remediation.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2022R1I1A3064380).
The author(s) declare(s) that there is no conflict of interest.
Harshavardhan Mohan: Investigation, Formal analysis, Writing - Review & Editing; Pavithra Muthukumar Sathya: Formal analysis, Writing - Original Draft; Min-Ju Kim:Data curation; Bongkyu Kim:Methodology, Formal analysis; Byung-Taek Oh: Funding acquisition & Project administration.
- 1. Almatouq, A., Babatunde, A. O., Khajah, M., Webster, G., and Alfodari, M. (2020). Microbial community structure of anode electrodes in microbial fuel cells and microbial electrolysis cells. Journal of Water Process Engineering, 34, 101140.
-

- 2. Chinnasamy, C., Perumal, N., Choubey, A., and Rajendran, S. (2023). Recent advancements in MXene-based nanocomposites as photocatalysts for hazardous pollutant degradation - A review. Environmental Research,233, 116459.
-

- 3. Costa, F., Lago, A., Rocha, V., Barros, Ó., Costa, L., Vipotnik, Z., Silva, B., and Tavares, T. (2019). A Review on Biological Processes for Pharmaceuticals Wastes Abatement—A Growing Threat to Modern Society. Environmental Science & Technology, 53(13), 7185-7202.
-

- 4. Idris, M. O., Kim, H.-C., Yaqoob, A. A., and Ibrahim, M. N. M. (2022). Exploring the effectiveness of microbial fuel cell for the degradation of organic pollutants coupled with bio-energy generation. Sustainable Energy Technologies and Assessments, 52, 102183.
-

- 5. Kong, W., Li, Y., Zhang, Y., and Liu, H. (2023). Enhanced degradation of refractory organics by bioelectrochemical systems: A review. Journal of Cleaner Production, 423, 138675.
-

- 6. Mahgoub, S. A., Qattan, S. Y. A., Salem, S. S., Abdelbasit, H. M., Raafat, M., Ashkan, M. F., Al-Quwaie, D. A., Motwali, E. A., Alqahtani, F. S., and Abd El-Fattah, H. I. (2023). Characterization and Biodegradation of Phenol by Pseudomonas aeruginosa and Klebsiella variicola Strains Isolated from Sewage Sludge and Their Effect on Soybean Seeds Germination. In Molecules (Vol. 28).
-

- 7. Matamoros, V., García, J., and Bayona, J. M. (2008). Organic micropollutant removal in a full-scale surface flow constructed wetland fed with secondary effluent. Water Research, 42(3), 653-660.
-

- 8. Moghiseh, Z., and Rezaee, A. (2021). Removal of aspirin from aqueous solution using electroactive bacteria induced by alternating current. Environmental Science and Pollution Research, 28(20), 25327-25338.
-

- 9. Moghiseh, Z., Rezaee, A., Ghanati, F., and Esrafili, A. (2019). Metabolic activity and pathway study of aspirin biodegradation using a microbial electrochemical system supplied by an alternating current. Chemosphere, 232, 35-44.
-

- 10. Mohan, H., Lim, J.-M., Cho, M., Park, Y.-J., Seralathan, K.-K., and Oh, B.-T. (2020). Remediation of BTEX and Cr(VI) contamination in soil using bioelectrochemical system—an eco-friendly approach. Environmental Science and Pollution Research, 27(1), 837-845.
-

- 11. Mohan, H., Sathya, P. M., Acharya, S., Jeong, H.-J., Lee, G.-M., Park, J.-H., Seralathan, K.-K., and Oh, B.-T. (2024). Harnessing Landfill-Derived Bacillus subtilis (LLS-04) for Bio-electrodegradation of Dibutyl Phthalate: Comprehensive Toxicity Assessment Across Multiple Biological Models. Journal of Hazardous Materials, 136480.
-

- 12. Mohan, H., Yoo, S., Thimmarayan, S., Oh, H. S., Kim, G., Seralathan, K.-K., and Shin, T. (2021). Nickel decorated manganese oxynitride over graphene nanosheets as highly efficient visible light driven photocatalysts for acetylsalicylic acid degradation. Environmental Pollution, 289, 117864.
-

- 13. Muthukumar Sathya, P., Mohan, H., Park, J.-H., Seralathan, K.-K., and Oh, B.-T. (2023). Applied potential assisted biodegradation of amoxicillin (AMX) using bacterial consortium isolated from a waste dump site. Chemosphere, 343, 140230.
-

- 14. Narayanan, M., El-sheekh, M., Ma, Y., Pugazhendhi, A., Natarajan, D., Kandasamy, G., Raja, R., Saravana Kumar, R. M., Kumarasamy, S., Sathiyan, G., Geetha, R., Paulraj, B., Liu, G., and Kandasamy, S. (2022). Current status of microbes involved in the degradation of pharmaceutical and personal care products (PPCPs) pollutants in the aquatic ecosystem. Environmental Pollution, 300, 118922.
-

- 15. Park, S.-R., Kim, S.-R., Min, E.-K., Oh, B.-C., Jung, Y., Kim, Y. H., & Lee, H.-Y. (2023). Unveiling the potential effects of acetylsalicylic acid: insights into regeneration in endometrial stem cells. Cell Communication and Signaling, 21(1), 323.
-

- 16. Poddar, K., Sarkar, D., Chakraborty, D., Patil, P. B., Maity, S., and Sarkar, A. (2022). Paracetamol biodegradation by Pseudomonas strain PrS10 isolated from pharmaceutical effluents. International Biodeterioration & Biodegradation, 175, 105490.
-

- 17. Rocheleau, H., Al-Harthi, R., and Ouellet, T. (2019). Degradation of salicylic acid by Fusarium graminearum. Fungal Biology, 123(1), 77-86.
-

- 18. Saravanan, A., Kumar, P. S., Hemavathy, R. V., Jeevanantham, S., Harikumar, P., Priyanka, G., and Devakirubai, D. R. A. (2022). A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Science of the Total Environment, 812, 152456.
-

- 19. Sathya, P. M., Mohan, H., Park, J.-H., Seralathan, K.-K., Cho, M., and Oh, B.-T. (2024). Bio-electrochemical degradation of carbamazepine (CBZ): A comprehensive study on effectiveness, degradation pathway, and toxicological assessment. Journal of Environmental Management, 360, 121161.
-

- 20. Sathya, P. M., Mohan, H., Park, J.-H., Seralathan, K.-K., and Oh, B.-T. (2024). Integrated bio-electrochemical approach to Norfloxacin (NFX) degradation: Efficacy, degradation mechanisms, and toxicological insights. Chemosphere,366, 143479.
-

- 21. Sathya, P. M., Mohan, H., Venkatachalam, J., and Seralathan, K.-K. (2023). A hybrid technique for sulfamethoxazole (SFM) removal using Enterobacter hormaechei HaG-7: Bio-electrokinetic degradation, pathway and toxicity. Chemosphere,313, 137485.
-

- 22. Shangguan, J., Yang, N., Zhang, L., Liu, J., Xia, X., and Xu, B. (2025). Employing Chlorella pyrenoidosa in eco-friendly acetylsalicylic acid degradation: Insights from physiology and transcriptomics. Bioresource Technology, 428, 132444.
-

- 23. Tavker, N., and Kumar, N. (2023). Chapter 6 - Bioelectrochemical systems: Understanding the basics and overcoming the challenges. In M. P. Shah, S. Rodriguez-Couto, A. Kumar Nadda & A. Daverey (Eds.), Development in Wastewater Treatment Research and Processes (pp. 79-98): Elsevier.
-

- 24. Wang, F., Xiang, L., Leung, K. S.-Y., Elsner, M., Zhang, Y., Guo, Y., Pan, B., Sun, H., An, T., and Ying, G. (2024). Emerging contaminants: a one health perspective. The Innovation.
- 25. Wang, H., Xi, H., Xu, L., Jin, M., Zhao, W., and Liu, H. (2021). Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Science of the Total Environment, 788, 147819.
-

- 26. Wang, H., and Zhou, Q. (2024). Bioelectrochemical systems – A potentially effective technology for mitigating microplastic contamination in wastewater. Journal of Cleaner Production, 450, 141931.
-

- 27. Wang, S., Hadji-Thomas, A., Adekunle, A., and Raghavan, V. (2024). The exploitation of bio-electrochemical system and microplastics removal: Possibilities and perspectives. Science of the Total Environment, 172737.
-

 This Article
This Article
-
2025; 30(6): 109-117
Published on Dec 31, 2025
- 10.7857/JSGE.2025.30.6.109
- Received on Nov 27, 2025
- Revised on Dec 12, 2025
- Accepted on Dec 23, 2025
 Services
Services
- Abstract
1. introduction
2. materials and methods
3. results
4. discussion
5. conclusion
- Acknowledgements
- Conflicts of Interest
- Author Contributions
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Bongkyu Kim, and Byung-Taek Oh
-
Division of Biotechnology, Advanced Institute of Environment and Bioscience, College of Environmental and Bioresource Sciences, Jeonbuk National University, Iksan, Jeonbuk State 54596, Republic of Korea
- E-mail: bkim@jbnu.ac.kr, btoh@jbnu.ac.kr





